Is Aging Due To Loss Of Dna Repair?
Abstract
Crumbling is a complex process that results in loss of the ability to reattain homeostasis following stress, leading, thereby, to increased adventure of morbidity and mortality. Many factors contribute to aging, such equally the time-dependent accumulation of macromolecular impairment, including DNA impairment. The integrity of the nuclear genome is essential for cellular, tissue, and organismal health. Dna damage is a abiding threat because nucleic acids are chemically unstable under physiological atmospheric condition and vulnerable to attack by endogenous and environmental factors. To combat this, all organisms possess highly conserved mechanisms to detect and repair Dna damage. Persistent DNA damage (genotoxic stress) triggers signaling cascades that bulldoze cells into apoptosis or senescence to avoid replicating a damaged genome. The drawback is that these cancer abstention mechanisms promote aging. Hither, nosotros review bear witness that Dna damage plays a causal office in aging. Nosotros also provide testify that genotoxic stress is linked to other cellular processes implicated as drivers of crumbling, including mitochondrial and metabolic dysfunction, altered proteostasis and inflammation. These links betwixt damage to the genetic code and other pillars of aging support the notion that Dna damage could be the root of aging.
Introduction
Aging is a multifactorial process that results in increased risk of a myriad of chronic diseases. Existence elderly is the greatest risk cistron, by orders of magnitude, for cancer, osteoporosis, cardiovascular disease, dementia and most other degenerative diseases (Kirkwood, 2005). While no single mechanism or pathway fully accounts for historic period-associated functional decline, one prevailing theory is that macromolecular damage, accumulating over fourth dimension, plays a causal role in driving aging. Most macromolecules in the cell when damaged are but degraded and replaced. In contrast, the nuclear genome, which is the blueprint for all cellular functions, has dedicated and energetically costly repair mechanisms to rapidly right Dna damage. This intimates that DNA impairment is a particularly chancy type of macromolecular damage and therefore likely to be deleterious to cellular homeostasis.
Maintaining genome stability is a continuous process. Deoxyribonucleic acids are chemically unstable under physiological conditions (aqueous, oxygen-rich, and pH seven.four) (Lindahl, 1993). DNA is also vulnerable to chemical attack past electrophiles and free radicals. While exogenous sources of genotoxic stress tin exist quite potent, endogenous threats are constant and relentless (Table 1). The nigh common Dna lesion is hydrolytic cleavage of the glycosidic bond betwixt the DNA base and sugar phosphate grouping, leading to abasic sites. Hydrolytic deamination of the DNA bases is also common. Products of normal cellular metabolism tin cause oxidation, nitrosylation, and alkylation of the Deoxyribonucleic acid bases (De Bont and van Larebeke, 2004). Breaks in the phosphate deoxyribose backbone ascend as a event of high energy radiation or during Deoxyribonucleic acid metabolism (replication, decatenation). Spontaneous DNA damage occurs on the order of 104–10v events per cell per day (Lindahl, 1993; De Bont and van Larebeke, 2004).
Estimated frequencies of DNA lesions caused by endogenous and mutual environmental sources of DNA damage.
Adjusted from Friedberg, 2006; Lindahl, 1993; Sander et al., 2005; Sears and Turchi, 2012; Mouret et al., 2006.
| Endogenous Dna adducts | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DNA lesion | DSB | Cytosine deamination | Cyclopurine adducts | Depyrimidination | eight-oxoG | Malondialdehyde adducts | Alkylation adducts | Depurination | SSB |
| Frequency per cell per day | teni | 102 | tentwo | ten2 | teniii | 103 | 103 | xiv | 104 |
| DNA adducts caused past environmental exposures | |||||||||
| Genotoxin | Sunlight | Background radiation | Ionizing radiations therapy | Oxaliplatin cancer therapy | |||||
| Lesion | Photodimers | Damaged bases | SSB | DSB | Damaged bases | SSB | Intra- and interstrand crosslinks | ||
| Frequency per cell per day | 102 in peel cells but | 10 | 2–5 | 0.25 | tenthree | 103 | 10three | ||
Dna is also susceptible to damage past environmental factors such as ultraviolet (UV), ionizing radiation, and alkylating agents used to care for proliferative disorders like cancer (Tabular array ane). Notably, even when exogenous genotoxin exposure is instigated with the purpose of driving cell decease (eastward.m., in cancer therapy) adduct burdens are well beneath the incidence of endogenous damage (Jackson and Loeb, 2001). Fortunately, all organisms have robust mechanisms to sense all types of DNA damage, delay genome replication (if needed), signal for repair, and correct or tolerate the large number of genomic insults that occur on a daily basis (Hoeijmakers, 2009). DNA harm that is non repaired in a timely manner or is as well egregious to exist repaired induces signaling events that lead to one of many cell fates, one of which, senescence, plays a causal role in aging.
Conceptually, could Dna harm drive aging?
Why? and how? organisms historic period remain challenging questions. Why one ages interrogates the reasons. How one ages interrogates the method. The combative pleiotropic theory of aging provides a genetic solution to why nosotros age, posing that genes that provide an advantage during reproductive life are disadvantageous post reproduction, yet these genes cannot exist selected against (Kirkwood, 2005). As an example of antagonistic pleiotropy, activation of the DNA impairment response (DDR) is critical for preventing cancer; however, chronic activation of the DDR is thought to drive the accumulation of senescent cells and chronic sterile inflammation in erstwhile age, equally described in more particular beneath. The pillars of aging (Kennedy et al., 2014) describe the method by which (or how) we age: loss of or impaired, mitochondrial integrity and part, metabolism, stem cell function, proteostasis, food sensing, adaptation to stress, autophagic flux, epigenetic command, and an accumulation of damaged cellular macromolecules. This includes damage to the nuclear and mitochondrial genomes. Nevertheless, it has proven challenging to establish the pillars of aging as true causes of aging rather than merely consequences of aging.
If Dna impairment drives aging, mechanistically how does it practise then? Through activating signaling responses (d'Adda di Fagagna et al., 2003), blocking transcription (Vermeij et al., 2016) and other DNA metabolism, altering the epigenome (Oberdoerffer et al., 2008), mutagenesis (Vijg, 2014), triggering cells senescence or apoptosis? Deoxyribonucleic acid damage occurs stochastically but the amount and types of Dna harm i experiences is influenced by the expression of genes encoding antioxidant enzymes, genes linked to energetics and mitochondrial part, and a myriad of other factors such as histones, methylases, sirtuins, transcription, and replication factors. Every aspect of how DNA harm might drive crumbling is too genetically adamant via the cellular response to DNA damage. The somewhat surprising finding is that DNA impairment has far-reaching effects on many aspects of cellular metabolism tied to crumbling, the so-called pillars of aging (Kennedy et al., 2014). This suggests that aging might exist driven by many types of cellular damage yet does not occur until one reaches a state where multiple aspects of cell biological science are perturbed, for instance, genome integrity, proteostasis, and mitochondrial function.
DDR and cell fate decisions
In one case Deoxyribonucleic acid harm is recognized in the nuclear genome, bulky adducts, small-scale miscoding lesions, single-strand breaks, or non-complex double-strand breaks (DSBs) can be straight repaired by nucleotide excision repair (NER), base excision repair (BER), and non-homologous end-joining (NHEJ), respectively. If replication forks or transcription complexes encounter polymerase-blocking lesions (Vermeij et al., 2016), this can atomic number 82 to the formation of a DSB or R-loop (Tresini et al., 2015), which potently actuate signaling events that halt prison cell cycle progression and promote repair. If the impairment signals persist, so the jail cell selects a fate that avoids replicating a damaged genome and mutagenesis at all cost, by activating events that lead to prison cell death (apoptosis) or irreversible prison cell cycle abort (senescence) (Figure 1). Dna damage signaling begins with the MRE11-RAD50-NBS1 (MRN) circuitous activating the phosphatidylinositol iii-kinase-similar kinases (PIKKs) ataxia-telangiectasia mutated (ATM), ATM-related kinase (ATR) and/or related PIKK (Thompson, 2012). ATM is activated primarily by Deoxyribonucleic acid DSBs while ATR is primarily involved in the response to stalled replication forks, although overlap occurs. Initially ATM and ATR work with checkpoint mediator proteins like MDC1, 53BP1 and BRCA1, TOPBP1, which are sensor proteins that bind to lesions and recruit other DDR factors. Impairment recognition is followed past phosphorylation (and activation) of transducer kinases like checkpoint kinase two (CHK2) or checkpoint kinase ane (CHK1), which dilate the ATM-ATR signal. CHK2 activation leads to activation of p53, a master regulator of the cellular response to genotoxic stress (Senturk and Manfredi, 2013). Sustained activation of these transducer proteins leads to phosphorylation of several Deoxyribonucleic acid repair and cell wheel checkpoint proteins (Thompson, 2012; Cheng and Chen, 2010). p53 can likewise be activated independently of ATM. p14/ARF inhibits MDM2–p53 interaction, resulting in the stabilization of p53 (Senturk and Manfredi, 2013; Meek and Anderson, 2009). p53 tin can mount a bimodal response to stress where it functions to activate apoptosis via diverse transcriptional targets like Bax, Puma, and Noxa, or promote transient or prolonged cell cycle abort through transcriptional activation of the cyclin-dependent kinase inhibitor p21CIP1 (Reinhardt and Schumacher, 2012). A myriad of factors determines the extent of p53 activation in response to genotoxic stress, and more factors likely volition exist discovered.
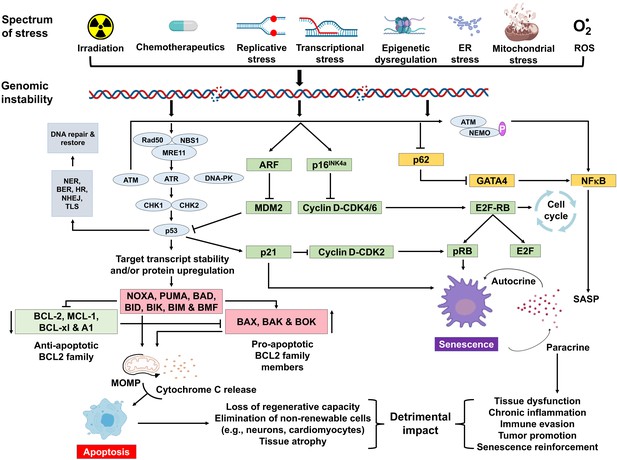
Schematic representation of signaling events inside a prison cell that enable DNA damage to promote aging.
Depicted are various stressors that can lead to genome instability and activation of the DNA damage response (DDR). The DDR (light blue) leads to jail cell cycle abort (green). If signaling persists, apoptosis or senescence ensues. Senescence tin can impact neighboring, undamaged cells.
A diversity of types of stress tin actuate the INK4a/ARF locus in cells. The factor products of the locus—p16INK4a and ARF—arrest cell progression by acting on the retinoblastoma protein and p53 (Senturk and Manfredi, 2013). p16INK4a inhibits CDK4 from binding to cyclin D, thereby preventing phosphorylation of RB. Hypo-phosphorylated retinoblastoma (RB) protein represses E2F-dependent gene expression, blocking G1/S cell cycle progression. Free E2F activates many genes involved in mitosis. While sensing Deoxyribonucleic acid damage and responding to information technology is a positive attribute, persistent DNA damage signaling can take quite deleterious effects. It can atomic number 82 to chronic activation of p53 and other response pathways (stress response, pro- and anti-apoptotic, pro-inflammatory, etc.), which touch on many aspects of cellular function and affect cell fate. Definitive cognition about which factors determine the selection of cell fate choice remains elusive. However, it seems likely that these factors may vary with the cell type, the level, and the elapsing of genotoxic stress.
Cellular senescence and the secretory phenotype
Leonard Hayflick and Paul Moorhead defined cellular senescence as the proliferative arrest that occurs in normal cells later a number of prison cell divisions (Hayflick and Moorhead, 1961; Hayflick, 1965). The starting time molecular explanation for senescence was the progressive telomere shorting that occurs with every prison cell division termed telomere-initiated cellular senescence (Harley et al., 1990). Many cells undergo senescence independently of telomere shorting due to replicative, mitochondrial, oxidative, metabolic, or genotoxic stress (Parrinello et al., 2003; Christoffersen et al., 2010; Correia-Melo et al., 2016; Nair et al., 2015). Senescent cells actively repress jail cell cycle progression preventing the replication of a damaged genome. Senescent cells take altered metabolism, morphology, and secretory profile called the senescence-associated secretory phenotype (SASP). The SASP includes pro-inflammatory cytokines, chemokines, and proteases (Coppé et al., 2008) and is heterogenous depending on the cell type and the inducer of senescence (Basisty et al., 2020). The SASP is thought to instigate immune clearance of the damaged/stressed cells (Tasdemir et al., 2016), but the immediate negative upshot is the potential to drive local tissue damage and systemic chronic sterile inflammation (Franceschi and Campisi, 2014).
I of the challenges of studying senescence is the absenteeism of a specific marking to identify senescent cells. Therefore, multiple endpoints must be measured simultaneously to identify them (Gorgoulis et al., 2019; Sharpless and Sherr, 2015). The well-nigh widely used marking is measurement of senescence-associated β-galactosidase (SA-β-gal) activity (Itahana et al., 2007). Other markers associated with cellular senescence include absence of proliferation markers (e.one thousand., Ki-67), increased expression of the cyclin-dependent kinase inhibitors p16INK4a and p21CIP1, persistent activation of the DDR (such as p53, ATM, ATR, ɣH2AX, telomere dysfunction-induced foci [TIF], senescence-associated heterochromatin foci [SAHF], and DNA segments with chromatin alterations reinforcing senescence [DNA-SCARS]), reduced lamin B1 expression, increased HMGB1 and SASP factors (such as IL-1β, IL-six, IL-10, MCP-1, and TNFα) (Coppé et al., 2010; Lawless et al., 2010; Serrano et al., 1997; Takai et al., 2003; Matjusaitis et al., 2016; Rodier et al., 2011).
Cellular senescence plays a critical role in preventing tumorigenesis, tissue repair, and wound healing, promoting insulin secretion and mammalian development (Kuilman et al., 2008; Krizhanovsky et al., 2008; Demaria et al., 2014; Storer et al., 2013; Helman et al., 2016). Notwithstanding, time-dependent accumulation of senescent cells, likely through decreased immunoclearance, occurs in virtually all vertebrates (Jeyapalan et al., 2007; Liu et al., 2009) and has been definitively shown to drive aging (Baker et al., 2016), rather than just occur with aging. Senescent cells also contribute to age-related diseases such as atherosclerosis (Roos et al., 2016), osteoporosis (Chandra et al., 2020), nonalcoholic fatty liver disease (Ogrodnik et al., 2017), cancer (Alimirah et al., 2020), neurodegenerative diseases (Chinta et al., 2018; Zhang et al., 2019), and numerous other age-related conditions (Kirkland and Tchkonia, 2020). Interestingly, NF-κB is the transcription factor that is most activated with aging (Adler et al., 2007). NF-κB signaling is the major signaling pathway that stimulates SASP (Tilstra et al., 2011). Dna harm activates NF-κB signaling via an ATM-NEMO-dependent regulation of an upstream kinase (Miyamoto, 2011; Figure 1). DNA impairment can as well lead to NF-κB activation via stabilization of GATA4 (Kang et al., 2015). Thus, genotoxic stress is a potent inducer of senescence and SASP, key drivers of crumbling and age-related disease.
Accelerated aging in genome instability syndromes
The mammalian genome encodes over 150 proteins directly responsible for safeguarding its integrity (Friedberg, 2006; Wood et al., 2005). These cistron products constantly monitor the quality and repair the nuclear genome (Sancar et al., 2004). Distinct DNA repair pathways cope with different types of DNA lesions: BER for small covalent additions to Dna bases, NER for bulky adducts that disrupt the Deoxyribonucleic acid helix, interstrand crosslink (ICL) repair to remove covalent links between the two strands of DNA, NHEJ to ligate broken Deoxyribonucleic acid ends, mismatch repair to correct replication errors, homologous recombination to manage replication stress and DSBs non readily ligated. Inherited defects in each of the Dna repair pathways are linked to distinct genome instability syndromes (Table ii). Broadly, the syndromes are characterized by developmental defects, increased incidence of cancer and features of accelerated aging (Cleaver, 1968; Menck and Munford, 2014; Wood, 2018). Progeroid syndromes are diseases of dramatically accelerated aging and include Hutchinson-Gilford, Werner, and Cockayne syndromes (CS), as well as XFE progeroid syndrome, all of which are linked to genome instability (de Magalhães, 2005; Martin and Oshima, 2000; Burla et al., 2018). The syndromes provide an elegant yet tragic glimpse into the touch that Dna damage tin can have on human wellness. Most syndromes were described well earlier Dna had been discovered or mechanisms of repair described.
Human genome instability diseases with historic period-associated symptoms.
| Disease | Afflicted genome stability pathway | Mutated genes | Crumbling-associated symptoms | Ref(due south) |
|---|---|---|---|---|
| Hutchinson-Guilford progeria syndrome | Chromatin organization | LMNA | Alopecia, atherosclerosis, arthritis, cardiovascular affliction, lipodystrophy, osteoporosis, skin aging and atrophy | Kudlow et al., 2007; Liu et al., 2005 |
| Nestor-Guillermo progeria syndrome | Chromatin organization | BANF1 | Baldness, atherosclerosis, arthritis, cardiovascular disease, lipodystrophy, osteoporosis, and pulmonary hypertension | Cabanillas et al., 2011; Loi et al., 2016 |
| Werner syndrome | Telomeric maintenance and replication stress | WRN | Alopecia, atherosclerosis, arthritis, cardiovascular disease, cataracts, diabetes, sarcopenia, and increased risk of cancer | Kudlow et al., 2007; Sugimoto, 2014 |
| Rothmund-Thomson syndrome | Dna replication initiation | RECQL4 | Alopecia, cataracts, osteoporosis, skin atrophy, and increased chance of cancer | Croteau et al., 2012; Ghosh et al., 2012 |
| Blossom syndrome | DNA replication and recombination | BLM | Diabetes, pulmonary illness, increased risk of cancer | Hanada and Hickson, 2007; de Renty and Ellis, 2017 |
| XFE progeroid syndrome | NER, ICL, and DSB repair | ERCC4 | Anemia, cardiovascular disease, kidney disease, neurodegeneration, osteoporosis, sarcopenia, sensory loss, and pare atrophy | Niedernhofer et al., 2006 |
| Xeroderma pigmentosum | NER and translesion Deoxyribonucleic acid synthesis | XPA-G, XPV | Premature pare photoaging, neurodegeneration, and increased incidence of skin cancer | Lehmann et al., 2011; Kraemer and DiGiovanna, 2015 |
| Cockayne syndrome | Transcription-coupled NER | CSA, CSB, XPB, XPD, XPG | Ataxia, cataracts, musculus cloudburst, and neurodegeneration | Nance and Drupe, 1992; Wilson et al., 2016 |
| Trichothiodystrophy | Transcription-coupled NER | TTDA, TTDN1, XPB, XPD | Premature bone marrow exhaustion and increased risk of cancer | Faghri et al., 2008; de Boer et al., 2002 |
| Fanconi anemia | ICL repair | FANCA-FANCW | Premature bone marrow exhaustion and increased risk of cancer | Ceccaldi et al., 2016; Nalepa and Clapp, 2018 |
| Ataxia telangiectasia | Deoxyribonucleic acid damage response | ATM | Premature bone marrow exhaustion, diabetes, and neurodegeneration | Rothblum-Oviatt et al., 2016 |
| Mandibular hypoplasia, deafness, progeroid features, lipodystrophy syndrome | Mail service-replication repair and translesion DNA synthesis | POLD1 | Diabetes, lipodystrophy, osteoporosis, steatosis, sensory loss | Weedon et al., 2013 |
| Ruijs-Aalfs syndrome | Poly peptide-Dna crosslink repair | SPRTN | Baldness, atherosclerosis, cataracts, diabetes, premature graying of hair, osteoporosis, sarcopenia, and increased risk of cancer | Lessel et al., 2014 |
| Alpers-Huttenlocher syndrome | Mitochondrial DNA replication and repair | POLG1 | Progressive neurodegeneration and liver disease | Nguyen et al., 2006 |
Children with Hutchinson-Gilford progeroid syndrome (HGPS) develop many features of premature aging in the kickoff decade of life including alopecia, atherosclerosis, osteolysis, and lipodystrophy among others (Hennekam, 2006). The genetic cause for HGPS are mutations in the LMNA gene, which encodes critical components of the nuclear lamina (Eriksson et al., 2003; De Sandre-Giovannoli et al., 2003) causing pernicious alterations in nuclear architecture resulting in genome instability (Kudlow et al., 2007). Nestor-Guillermo progeroid syndrome, caused by mutations in the nuclear lamina factor BANF1, has characteristics of accelerated aging due to impaired chromatin organization (Cabanillas et al., 2011; Loi et al., 2016).
Mutations in Deoxyribonucleic acid helicases are linked to multiple diseases of accelerated crumbling. Mutations in three of the RECQ family unit of helicases (BLM, RECQL4, and WRN) cause progeroid syndromes (Uchiumi et al., 2015). Mutations in WRN, a factor that encodes a helicase responsible for managing replication stress and telomere stability, cause Werner syndrome (WS) (Kudlow et al., 2007). WS patients feel growth retardation, premature hair graying, lipodystrophy, besides as premature onset of multiple historic period-related diseases including arteriosclerosis, cancer, type 2 diabetes mellitus, and osteoporosis (Sugimoto, 2014). Rothmund-Thomson syndrome (RTS) is caused past mutations in RECQL4, encoding a helicase that participates in DNA replication and repair. RTS patients experience juvenile cataracts, epidermal cloudburst, and increased cancer incidence (Croteau et al., 2012; Ghosh et al., 2012). Bloom syndrome (BS) is caused by mutations in BLM, which encodes a RecQ helicase critical for suppressing recombination and thereby genome instability (Nguyen et al., 2014; Hanada and Hickson, 2007). The mean lifespan of BS patients is 26 years and they take premature onset of numerous historic period-related diseases, including cancer, diabetes, and chronic obstructive pulmonary disease (de Renty and Ellis, 2017).
NER detects and removes Deoxyribonucleic acid adducts that distort the helical structure of DNA such as adducts resulting from exposure to UV light, environmental mutagens, and certain classes of cancer chemotherapeutics (Schärer, 2013). Endogenous DNA adducts repaired past NER are cyclopurines; oxidative DNA lesions believed to contribute to neurodegeneration (Brooks, 2008). NER consists of two sub-pathways that are responsible for repair of the entire nuclear genome (global genome NER) or focused on transcribed genes (transcription-coupled NER). Defects in the former crusade xeroderma pigmentosum (XP) linked to mutations in vii genes XPA-G (Cleaver, 1968; Lehmann et al., 2011). XP is characterized by accelerated photoaging of the skin and a x,000-fold increased take chances of skin cancer. XP patients also oftentimes have accelerated onset of peripheral neuropathy, sensorineural deficits, cerebral atrophy, and neurodegeneration (Kraemer and DiGiovanna, 2015). Defects in transcription-coupled NER cause CS or trichothiodystrophy (TTD). CS patients have progressive growth retardation, neurodegeneration, cataracts, osteoporosis, and metabolic dysfunction like to old age (Nance and Drupe, 1992; Wilson et al., 2016). TTD patients present with CS-like features plus brittle hair and ichthyosis (Faghri et al., 2008). XFE progeroid syndrome results from mutations in ERCC4/FANCQ causing reduced expression of XPF-ERCC1, a heterodimeric DNA repair endonuclease required for NER, DNA ICL repair, and the repair of some Deoxyribonucleic acid DSBs (Niedernhofer et al., 2006; Ahmad et al., 2008; Bhagwat et al., 2009). The picket XFE patient, who presented with exceptional photosensitivity and exhibited clear signs of premature aging of near all organ systems, lived to but sixteen years of age (Niedernhofer et al., 2006).
DNA ICLs are incredibly genotoxic lesions that impair transcription and replication by compromising DNA strand separation (Clauson et al., 2013). ICLs tin be caused by endogenous metabolites (e.g., by-products of lipid peroxidation) or environmental exposures (east.1000., bifunctional cancer chemotherapeutics such as cisplatin). ICLs are recognized past the Fanconi anemia (FA) repair pathway in replicating cells and past NER in non-dividing cells and are removed past endonucleases in these pathways (Ceccaldi et al., 2016). Defects in ICL repair cause FA, a rare affliction in which patients showroom accelerated bone marrow failure, increased risk of, congenital defects, endocrine dysfunction and other aging features (Nalepa and Clapp, 2018).
Mutations in the ATM, which encodes a serine/threonine kinase activated in response to Dna damage, cause ataxia telangiectasia (AT) (Thompson, 2012; Valentin-Vega et al., 2012). AT patients develop dysphagia, dysphonia, loss of motor control, diabetes as adolescents, premature crumbling of the hair and peel, consistent with accelerated aging (Rothblum-Oviatt et al., 2016). Mutations in POLD1 and SPRTN cause two Werner-like progeroid syndromes: mandibular hypoplasia, deafness, progeroid features, lipodystrophy syndrome, and Ruijs-Aalfs syndrome, respectively (Weedon et al., 2013; Lessel et al., 2014). Alpers-Huttenlocher syndrome, marked by progressive neurodegeneration and liver failure, is caused by mutation in the mitochondrial Dna polymerase politician ɣ (Nguyen et al., 2006). Clinically, these syndromes are distinct. The distinctions likely ascend from how disquisitional a item Dna repair mechanism is for protecting the integrity of a specific cell type or tissue. Yet there are also tremendous commonalities in the pathophysiology of the distinct syndromes, and the commonalties largely align with changes associated with normal aging. It is humbling to recognize that the vast majority of symptoms seen in these genome instability disorders is driven by endogenous Dna damage.
It is important to note that while the focus here is on human repair-deficiency phenotypes, Deoxyribonucleic acid repair and response genes are for the most part highly conserved. Murine models of these genome instability disorders largely recapitulate the human disorders and take been extremely valuable for discerning genotype:phenotype correlations (Friedberg and Meira, 2006). Studies in patient cells, yeast, and even bacteria were all essential for establishing the mechanisms of Dna repair also signaling mechanisms in response to unrepaired Deoxyribonucleic acid damage, which contributes more potently to driving aging than the harm itself.
Iatrogenic genotoxins drive aging
The number of cancer survivors is increasing dramatically every bit a outcome of improved therapeutics (Bluethmann et al., 2016). Unfortunately, then is the awareness that those treated with genotoxic agents are aging rapidly (Hurria et al., 2016). Radiation and genotoxic chemotherapy are used to impale rapidly dividing cells, like tumor cells. However, they are non specific for cancer cells. All cells in the trunk feel genotoxic stress during therapy and this triggers signaling events and jail cell fate decisions that appear to advance aging. Cancer survivors display premature onset of frailty and multi-morbidities associated with old historic period, often decades before than expected (Killock, 2014). For instance, 20% of babyhood cancer survivors have ischemic heart illness or stroke by 50 years of age compared to i% incidence for their siblings (Armenian et al., 2018). Whether DNA impairment is increased by exposure to genotoxins or by genetic depletion of repair mechanisms, the consequence is the aforementioned: accelerated aging. This supports the notion that DNA harm, regardless of whether the source is endogenous or environmental, tin can be both the why and how aging occurs.
It is at present well documented that chemotherapy with anthracycline, a DNA intercalating agent used for treating breast cancer, causes a significant increase in the expression of p16INK4a in peripheral T cells, a recognized measure out of crumbling (Liu et al., 2009; Sanoff et al., 2014). This increased p16INK4a expression persists for nearly a year after handling and equates to the rise in p16INK4a expression that occurs with 15 years of chronological crumbling (Sanoff et al., 2014). Pediatric cancer survivors take a >ii.5-fold increased incidence of disabling age-related diseases compared to their siblings by 50 years of age (Armstrong et al., 2014). This includes arthritis, cardiovascular disease, kidney, and pulmonary affliction. Platinum-based therapy (causing intra- and interstrand DNA crosslinks) causes a significantly increased risk of cardiovascular disease and pre-diabetes (Baker, 2013). A key tenet of aging is frailty, which is defined as disability, reduced endurance, risk of falls, and hospitalization due to chronic diseases that typically occur at the end of life (Vetrano, 2018). Ten pct of individuals ≥65 years sometime are fragile. Remarkably this is duplicate from the incidence of frailty in childhood cancer survivors in their thirties (Ness et al., 2015). Performance on physical role tests (walking and grip strength) is the same for individuals >65 years of age and survivors of pediatric cancers who are decades younger (Henderson et al., 2014). The accelerated senescence and crumbling observed in cancer survivors specifically treated with genotoxic agents strongly supports the view that DNA harm drives aging.
Dna damage impacts every aspect of cell biology
Genotoxic stress is a strong driver of cellular senescence and senescent cells play a causal role in driving aging and age-related affliction. What is truly remarkable is looking inside cells harboring genotoxic stress and finding how greatly cellular homeostasis is perturb (Effigy ii). For case, in tissues of DNA repair-deficient mice (acquired by reduced expression of XPF-ERCC1 due to genetic depletion of Ercc1), spontaneous oxidative DNA damage accumulates rapidly, albeit not to a greater extent than what occurs with aging in repair-adept mice (Wang et al., 2012). As a consequence of this genotoxic stress, mitochondrial and metabolic dysfunction, and increased reactive oxygen species ascend, identical to changes seen with normal aging (Robinson et al., 2018). Interestingly, in the same Ercc1 mutant mice, which model XFE progeroid syndrome, senescent cells accrue in the same organs as occurs with normal aging in mice and to roughly the same extent (Yousefzadeh et al., 2020). The profound similarities between Deoxyribonucleic acid repair-deficient mice and aged mice support the notion that DNA damage drives aging. But more chiefly, the cellular perturbations downstream of DNA damage reinforces 'how' DNA damage drives aging: by perturbing every attribute of prison cell biological science. The consistency and uniformity with which these cellular responses to genotoxic stress occur argues that the changes are driven via signaling non as a event of random mutation or loss of transcription of key genes. Much remains to be elucidated nigh these signaling mechanisms. It is likewise interesting to note that the increased ROS observed in tissues of ERCC1-XPF mice is pathological and causes more DNA damage (Robinson et al., 2018), raising the signal that endogenous DNA impairment, if not repaired, becomes amplified.
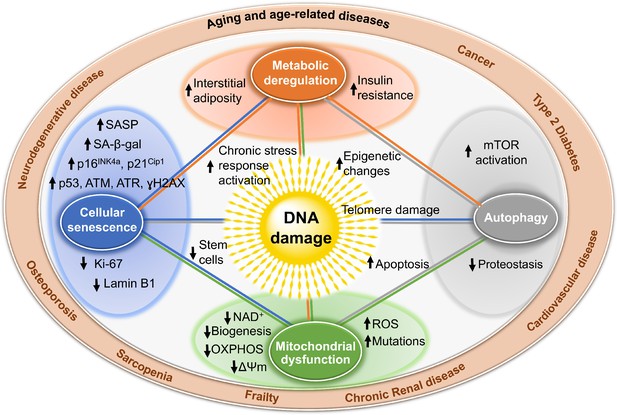
Mechanisms by which Dna damage could promote aging.
Deoxyribonucleic acid damage, including damage at telomeres (center), once detected, if not repaired, can interfere with replication or transcription, resulting in the activation of signaling events that modify cell physiology. Ane issue of these signaling events is apoptosis, which while depleting important cells similar stem cells or neurons may non be the near potent driver of aging. Dna damage can likewise outcome in mitochondrial dysfunction, impaired autophagy, metabolic changes, and the triggering of cellular senescence (pocket-size circles). These live but physiologically altered cells are predicted to be a more than potent commuter of crumbling and disease. Endpoints used to measure these consequences of DNA harm are indicated with arrows in the larger circles. These outcomes are all interconnected in that mitochondrial dysfunction can cause metabolic changes, impaired autophagy and proteostasis, more DNA damage, and senescence. This creates a cycle of increasing damage and dysfunction, which can spread to other cells via SASP, that is likely the proximal cause of aging and the diseases associated with it (outer circle).
Additional bear witness to back up the notion that genotoxic stress greatly perturbs cellular homeostasis includes the following. Cells from patients with XP, CS, and AT have altered energy homeostasis, impaired mitophagy, and an increased mitochondrial membrane potential, implying accumulation of dysfunctional mitochondria and increased ATP and oxygen consumption (Valentin-Vega et al., 2012; Scheibye-Knudsen et al., 2012; Fang et al., 2014). Poly(ADP-ribose) polymerase 1 (PARP1) is disquisitional for the detection and repair of strand breaks. Persistent activation of PARP1 depletes cellular reserves of nicotinamide adenine dinucleotide (NAD+) (Finkel et al., 2009), a disquisitional co-factor for many enzymes including sirtuins, which are a family unit of protein deacetylases and ADP-ribosyltransferases that broadly regulate gene expression and protein stability. SIRT1 also regulates mitochondrial biogenesis past deacetylating PGC-1α (peroxisome proliferator-activated receptor γ coactivator i α) (Finkel et al., 2009). SIRT1 activeness is dramatically reduced in beast models of XP and CS due to persistent activation of PARP1 (Scheibye-Knudsen et al., 2012; Fang et al., 2014). Inhibition of PARP1 or supplementation with NAD+ precursors restore SIRT1 activeness and better mitochondrial homeostasis and cellular metabolism (Scheibye-Knudsen et al., 2014; Fang et al., 2016).
Is more than Deoxyribonucleic acid repair beneficial?
The aureate standard for establishing a causal relationship between ii events (Dna damage and aging) is to demonstrate that impaired repair accelerates crumbling, while improved repair slows aging. This is tricky with DNA repair as in that location are no drugs that stimulate Dna repair, nor is it like shooting fish in a barrel to improve DNA repair genetically. Dna repair mechanisms require the coordinated action of numerous proteins. Overexpression of but i protein does not e'er improve repair and, in fact, can be detrimental (Shaposhnikov et al., 2015). All the same, in that location are some hints that longevity correlates with improved responses to genotoxic stress. In the nematode Caenorhabditis elegans, 40 single gene mutations accept been described that increase lifespan by at least 20% and in all cases these mutations confer resistance to UV irradiation (Johnson et al., 2002). Overexpression of human MTH1, which prevents 8-oxoG accumulation, in mice protects confronting neurodegeneration (De Luca et al., 2008) and extends lifespan (De Luca et al., 2013). Enhancing ATM activity in a murine model of HGPS reduces progeroid features and extends lifespan (Qian et al., 2018). Interspecies comparisons have not definitively identified a correlation betwixt Deoxyribonucleic acid repair capacity and lifespan (Austad, 2010; Cortopassi and Wang, 1996; Hart and Setlow, 1974), with a couple of exceptions. BER (but not NER) capacity is greater in cells from longer-lived rodents and non-homo primates, than in shorter-lived species (Austad, 2010). Naked mole rats (lifespan xxx+ years) take improved NER and BER efficiency relative to mice (lifespan 3 years) (Evdokimov et al., 2018). Many longer-lived species take increased expression or sequence optimization of key regulators of genome stability (Keane et al., 2015; Tian et al., 2017) leading to improved DSB repair in longer-lived mammals (Tian et al., 2019). Cells of centenarians accept improved Deoxyribonucleic acid repair activeness and antioxidant capacity compared to non-centenarians (Chevanne et al., 2003; Franzke et al., 2015a). Calorie restriction (CR) is the well-nigh successful intervention to extend lifespan and/or health bridge in organisms ranging from yeast to mammals, including non-human primates. CR has been demonstrated to decrease the abundance of DNA damaging reactive oxygen species (reviewed in Pamplona and Barja, 2006), thereby reducing oxidative DNA damage (reviewed in Heydari et al., 2007). Consistent with this is the observation that CR reduces transcriptional stress in DNA repair defective mice (Vermeij et al., 2016). However, there is besides bear witness that CR might improve Deoxyribonucleic acid repair, including BER, NER, and NHEJ (studies in rodents thoroughly reviewed in Heydari et al., 2007). In humans, there is bear witness that CR (Matt et al., 2016), dietary micronutrients (Ames, 2010), chronic practise, and improved socialization of the elderly (Franzke et al., 2015b) can enhance genome stability, which is ascribed to improved DNA repair capacity. Nevertheless, more studies using robust measures of Deoxyribonucleic acid repair capacity are needed. Collectively, there is arable evidence that more Deoxyribonucleic acid repair at least correlates with improved healthspan and lifespan.
Conclusions
In that location is now sufficient and diverse prove to support a cogent argument that Deoxyribonucleic acid damage plays a causal role in crumbling. This includes environmental/iatrogenic sources of genotoxic stress as well equally spontaneous/endogenous genotoxic stress. Dna damage contributes to aging via cell autonomous events such as causing apoptosis, which depletes functional cells such as neurons, and via jail cell non-autonomous mechanisms such every bit triggering senescence, which can negatively bear upon the function of neighboring, undamaged cells through their SASP. Downstream consequences of DNA damage impinge upon all of the other pillars of aging resulting in a state of self-perpetuating damage, which likely is the ultimate crusade of crumbling. Despite these broad consequences of genotoxic stress, there is also evidence that these consequences can be modulated through approaches aimed at slowing aging, including caloric brake, NAD+ supplementation, or ablating senescent cells. The field is still lacking tools to measure out DNA lesions and DNA repair chapters that are accessible to the broader inquiry community. Building such a tool kit would enable more precise decision of when (nether what circumstances) and where (in what organs) Deoxyribonucleic acid damage truly drives aging. It too might open new opportunities in precision medicine, enabling fine tuning of DNA damage and repair to, for case, ameliorate tumor ablation, slow the loss of irreplaceable cells, or optimize metabolism to promote repair.
References
-
Conference
Cancer handling as an accelerated aging process: cess, biomarkers, and interventions
American Club of Clinical Oncology Educational Book. American Lodge of Clinical Oncology. Almanac Meeting. pp. e516–e522.
- Google Scholar
Article and author information
Writer details
Funding
National Institutes of Health (P01 AG043376)
- Paul Robbins
- Laura Niedernhofer
National Institutes of Health (U01 ES029603)
- Laura Niedernhofer
National Institutes of Health (R56 AG059676)
- Laura Niedernhofer
National Institutes of Health (R01 AG063543)
- Paul Robbins
- Laura Niedernhofer
American Federation for Aging Research (Irene Diamond Fund/American Federation for Aging Research Postdoctoral Transition Award)
- Matt Yousefzadeh
National Institutes of Health (U19 AG056278)
- Paul Robbins
- Laura Niedernhofer
The funders had no role in study pattern, data drove and interpretation, or the determination to submit the piece of work for publication.
Acknowledgements
We thank Mariah Witt for helpful comments. This work was supported past NIH grants P01 AG043376, U01 ES029603, R56 AG059676, R01 AG063543, and U19 AG056278. MJY was supported by the Irene Diamond Fund/American Federation for Aging Research Postdoctoral Transition Honor.
Senior Editor
- Jessica K Tyler, Weill Cornell Medicine, United States
Reviewing Editor
- Matthew Simon, University of Rochester, United States
Publication history
- Received: September 9, 2020
- Accepted: Jan 15, 2021
- Version of Record published: January 29, 2021 (version 1)
Copyright
© 2021, Yousefzadeh et al.
This commodity is distributed under the terms of the Creative Eatables Attribution License, which permits unrestricted use and redistribution provided that the original writer and source are credited.
Metrics
-
- xi,200
- Page views
-
- 846
- Downloads
-
- 18
- Citations
Article citation count generated by polling the highest count across the following sources: Crossref, PubMed Fundamental, Scopus.
Download links
A 2-part list of links to download the article, or parts of the article, in various formats.
Downloads (link to download the article as PDF)
Download citations (links to download the citations from this article in formats compatible with diverse reference manager tools)
Open up citations (links to open the citations from this commodity in diverse online reference director services)
Source: https://elifesciences.org/articles/62852
Posted by: thompsoncongs1955.blogspot.com


0 Response to "Is Aging Due To Loss Of Dna Repair?"
Post a Comment